Rice Uses Light to Remotely Trigger Biochemical Reactions
Chemical processes can be activated by light without the need for bulk heating of a material through a process developed by researchers at Rice University.

Since Edison’s first bulb, heat has been a mostly undesirable byproduct of light. Now researchers at Rice University are turning light into heat at the point of need, on the nanoscale, to trigger biochemical reactions remotely on demand.
The method created by the Rice labs of Michael Wong, Ramon Gonzalez and Naomi Halas and reported today in the American Chemical Society journal ACS Nano makes use of materials derived from unique microbes – thermophiles – that thrive at high temperatures but shut down at room temperature.
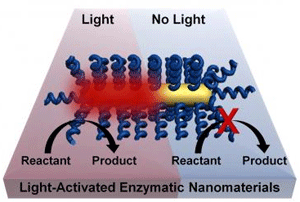
The Rice project led by postdoctoral fellow Matthew Blankschien and graduate student Lori Pretzer combines enzymes from these creatures with plasmonic gold nanoparticles that heat up when exposed to near-infrared light. That activates the enzymes, which are then able to carry out their functions.
This effectively allows chemical processes to happen at lower temperatures. Because heating occurs only where needed – at the surface of the nanoparticle, where it activates the enzyme – the environment stays cooler.
Blankschien thinks that’s fascinating.
“Basically, we’re getting the benefits of high-temperature manufacturing without needing a high-temperature environment,” said Blankschien, who won the Peter and Ruth Nicholas Postdoctoral Fellowship two years ago to work on these ideas. “The challenge was to keep the higher temperature at the nanoparticle, where the enzyme is activated, from affecting the environment around it.”
The technique holds great potential for industrial processes that now require heat or benefit from remote triggering with light.
“The implications are pretty exciting,” said Wong, a professor of chemical and biomolecular engineering and of chemistry. “In the chemical industry, there’s always a need for better catalytic materials so they can run reactions more inexpensively, more ‘green’ and more sustainably. You shouldn’t run through gallons of solvent to make a milligram of product, even if you happen to be able to sell it for a lot of money.”
For industry, the potential energy savings alone may make the Rice process worth investigating. “Here we’re using ‘free’ energy,” Wong said. “Instead of needing a big boiler to produce steam, you turn on an energy-efficient light bulb, like an LED. Or open a window.”
The particle at the center of the process is a gold nanorod about 10 nanometers wide and 30 long that heats up when hit with near-infrared light from a laser. The rods are just the right size and shape to react to light at around 800 nanometers. The light excites surface plasmons that ripple like water in a pool, in this case emitting energy as heat.
Halas’ Rice lab is famous for pioneering the use of gold nanoshells (a related material) to treat cancer by targeting tumors with particles that are bulk heated to kill tumors from the inside. The therapy is now in human trials.
The new research takes a somewhat different tack by heating nanoparticles draped with a model thermophilic enzyme, glucokinase, from Aeropyrum pernix. A. pernix is a microbe discovered in 1996 thriving near hot underwater vents off the coast of Japan. At around 176 degrees Fahrenheit, A. pernix degrades glucose, a process necessary to nearly every living thing. The enzyme can be heated and cooled repeatedly.
In their experiments, Blankschien and Pretzer cloned, purified and altered glucokinase enzymes so they would attach to the gold nanoparticles. The enzyme/nanoparticle complexes were then suspended in a solution and tested for glucose degradation. When the solution was heated in bulk, they found the complexes became highly active at 176 degrees, as expected.
Then the complexes were encapsulated in a gel-like bead of calcium alginate, which helps keeps the heat in but is porous enough to allow enzymes to react with materials around it. Under bulk heating, the enzymes’ performance dropped dramatically because the beads insulated the enzymes too well.
But when encapsulated complexes were illuminated by continuous, near-infrared laser light, they worked substantially better than under bulk heating while leaving the solution at near-room temperature. The researchers found the complexes robust enough for weeks of reuse.
“As far-fetched as it sounds, I think chemical companies will be interested in the idea of using light to make chemicals,” Wong said. “They’re always interested in new technologies that can help make chemical products more cheaply.”
He sees other possible uses for the new approach in the production of fuels from degradation of biomass like lignocellulose; for drug manufacture on demand – maybe from nanoparticle-infused tattoos on the body; or even for lowering blood sugar concentrations as a different way to manage diabetes.
“That we can now make these particles is great,” Wong said. “The next exciting part is in thinking about how we can deploy them.”
Ryan Huschka, a co-author of the paper, is a former Rice graduate student and now an assistant professor of chemistry at Newman University. Halas is the Stanley C. Moore Professor in Electrical and Computer Engineering, a professor of biomedical engineering, chemistry, physics and astronomy and director of Rice’s Laboratory for Nanophotonics. Gonzales is an associate professor of chemical and biomolecular engineering and also of bioengineering.
The research was supported by the Peter and Ruth Nicholas Postdoctoral Fellowship Program administered by the Richard E. Smalley Institute for Nanoscale Science and Technology, the Rice University Institute of Biosciences and Bioengineering Hamill Innovations Award Program, the Rice University Faculty Initiatives Fund, the Robert A. Welch Foundation, the National Security Science and Engineering Faculty Fellowship, the Defense Threat Reduction Agency, the Air Force Office of Scientific Research and the National Science Foundation.